Product Cat. No.: GBS-003
For Research Use Only.
BCR/ABL gene fusion detection kit.
10 Tests/box.
This kit is mainly used for the detection of BCR/ABL gene fusion. The test samples are bone marrow cells suspected or diagnosed with leukemia patients (clinical or post-treatment patients) by clinical routine examination and used only for auxiliary diagnosis of the patient’s molecular typing.
Leukemia is a kind of malignant clonal disease of hematopoietic stem cells. Clonal leukemic cells proliferate and accumulate in bone marrow and other hematopoietic tissues, and infiltrate other non-hematopoietic tissues and organs, because of an uncontrolled proliferation, differentiation and apoptosis, and inhibition of the normal hematopoietic function. BCR/ABL gene is a common cytogenetic anomaly in patients with chronic myelocytic leukemia (CML). The BCR/ABL fusion gene can be found in 90% of CML patients, and the prognosis of the patients with BCR/ABL gene is poor.
This kit was validated against the BCR/ABL gene fusion detection performance only, and was not combined with the drug for clinical validation. This kit is only suitable for the detection of BCR/ABL gene fusion status, the test results are for clinical reference only and should not be used as the only basis for diagnosis. The clinician should make comprehensive judgment on the test results in combination with other clinical indicators.
Fluorescence in situ hybridization is a technique for direct detection of specific nucleic acids in cells. According to the principle of complementary bases pairing, a specific probe is complementary to a target sequence within the cell. The probe and target sequence can be clearly observed under fluorescence microscope and under appropriate excitation light, due to the probe fluorescence.
The kit uses orange fluorescein-labeled ABL probe and green fluorescein-labeled BCR probe. By in situ hybridization technique, the two probes bind to the target detection site. Normally (if BCR/ABL gene have not fused), two orange red signals and two green signals are shown under fluorescence microscope .When there is fusion, green and orange signals form by recombination a yellow fusion signal.
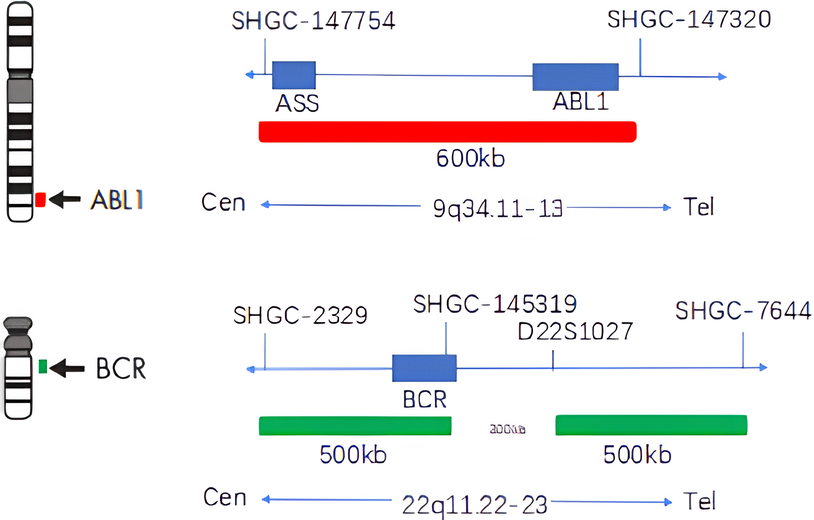
The kit consists of ABL orange probe and BCR green probe, as shown in Table 1. The reagents not provided in the kit are shown in Table 2.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| BCR/ABL dual color probe | 100μL/Tube | 1 | ABL orange probe, BCR green probe |
| Reagent name | Purity | Reagent name | Purity |
|---|---|---|---|
| Sodium chloride | Analytical purity AR | NP-40 | Analytical purity AR |
| Sodium citrate | Analytical purity AR | Xylene | Analytical purity AR |
| Anhydrous ethanol | Analytical purity AR | Protease K | ≥40 units/g |
Keep sealed away from light at -20oC±5oC. The product is valid for 12 months. Avoid unnecessary repeated freezing and thawing that should not exceed 10 times. After opening, within 24 hours for short-term preservation, keep sealed at 2~8oC in dark. For long-term preservation after opening, keep the lid sealed at -20oC±5oC away from light. See the label of the kit for the production date and expiration date.
1. Fluorescence microscopy imaging system includes fluorescence microscope and filtersets. The kit islabeled with orange fluorescein, and the filter set compatible with the fluorescent labeled dye should be selected.
Orange fluorescence: The maximum excitation wavelength is 552nm and the maximum emission wavelength is 576nm.
Green fluorescence: The maximum excitation wavelength is 496nm and the maximum emission wavelength is 520nm.
Fluorescence microscopy imaging system should use a microscope with orange and green channels. For monochromatic channel microscope, image synthesis analysis results should be used.
2. Automatic hybridization instrument: Strict temperature uniformity is required, and the temperature difference should be ≤1oC.
1. Applicable specimen types: Fresh specimen that have not been fixed stored at 4°C for less than 24 hours; Cell suspensions after fixation stored at -20°C for less than 6 months; Prepared cell slides stored at -20°C for less than 1 month.
2. When specimen are stored at too high or too low a temperature (eg, frozen), the specimen will not be used for testing and should be discarded.
3. If the cell suspension is excessively volatile or contaminated during storage, the sample should be discarded.
1. Related reagents
The following reagents are required for the experiment but not provided in this kit
Sodium chloride
Sodium citrate
176g
88g
Weigh 176g of sodium chloride and 88g of sodium citrate, dissolve in 800mL of deionized water, adjust the pH to 5.3±0.2 at room temperature, and complete to 1 L with deionized water. High-pressure steam sterilization, stored at 2~8oC, the solution shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
NP-40
0.6mL
20xSSC
4mL
Take 0.6mL NP-40 and 4mL 20×SSC, add 150mL deionized water, mix, adjust the pH to 7.0 ~ 7.5 at room temperature, with deionized water complete to a volume of 200mL. Stored at 2~8°C, the shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
2. Sample collection and Slides preparation
3. Slides pretreatment
4. Denaturation and Hybridization
The following operations should be performed in a darkroom.
5. Washing
The following operations should be performed in a darkroom.
6. Counterstaining
The following operations should be performed in a darkroom.
Dip 10μL of DAPI counterstain into the hybridization area of the glass slides, immediately cover and then use the appropriate filter to observe the sections under the fluorescence microscope.
7. FISH results observation
1. Common signal classification
| Signal type |
Diagram pattern ABL BCR |
Cells results determination |
|---|---|---|
Two orange red signals, two green |
|
Negative |
One orange signal, one green signal, |
|
Positive |
One orange signal, two green signals, |
|
Positive |
One orange signal, one green signal, |
|
Positive |
2. Threshold setting
Threshold should be set independently by each laboratory: Twenty human peripheral blood cell samples were randomly selected and processed according to sample processing requirements to prepare a deviant threshold reference slide. 200 cells were randomly counted in each reference slide. The number and percentage of cells that showed various types of positive cells were calculated, the mean and standard deviation of the statistical percentages were calculated.
The threshold A is 13.5% and the threshold B is 4%, can be used as a reference. Due to differences in sample processing methods and the subjective nature of signal counts, the laboratory thresholds will be different. Each laboratory should establish thresholds strictly in accordance with the standard process set by the threshold.
1. FISH results determination
After counting 200 cells, the number and percentage of various types of positive cells were calculated respectively,
2. Judgment of invalid experiment
| Question | Possible cause | Recommended solution |
|---|---|---|
|
Too strong background |
Inadequate washing after hybridization. Improper use of filter sets. Improper hybridization conditions. Low washing temperature. |
Ensure that the washing solution is prepared according to the instructions, ensure the correct pH value and temperature of the washing solution, remove the coverslip and repeat the washing steps. Replace the appropriate filter setsfor observation and to weaken the background light.Ensure that the hybridization instrument temperature is 45°C. Ensure that the hybridization instrument temperature is 45°C. Ensure that the solution temperature is at the washing slides required temperature. |
|
Too weak dye |
Too weak dye. Obsolete dye agent or excessive illumination |
68°C 0.3%NP-40/0.4 × In SSc solution,shake for 10 ~ 20 seconds, remove the cover glass and soak for 2 minutes. Place the slide in deionized water at 37 °C and soak it for 1 minute. Dry the slide naturally in the dark and then re dye it. Ensure that the dye agent is stored at -20°C and keep away from light. Make sure that the dye agent is valid. |
|
No signal or weak signal |
Specimen incomplete denaturation. Improper pre-denaturation specimens’ preparation. Probes and hybridization buffer improper mixture before usage. Probe mixture on the slide dries too fast Bubbles formation under coverslips during hybridization. Inappropriate hybridization conditions. Improper washing solution or washing conditions. Probe or specimen slides inadequate storage. Incorrect dye agent or too bright dye agent usage. The selected filter sets is unsuitable for observation. |
Ensure that the hybridization instrument temperature is at 88°C, and the hybridization instrument should be preheated at least 10min ahead of time. Please refer to the above sample preparation related questions and answers. Mix well the probe and the hybridization buffer, centrifuge briefly. Wash the coverslip in the washing solution. When covering the coverslip, cover the surface of the probe mix and squeeze gently to allow the bubbles to escape. Ensure to observe time and temperature specified for the hybridization; do not leave gapsin the rubber seals; adjust hybridization time as appropriate. Ensure that the washing solution is prepared according to the product specification; Check that the washing solution temperature reaches the in the washing step specified temperature; Assure that the thermometer and the pH meter are correctly calibrated. Ensure that the probe is stored in dark at -20°C. Place the unhybridized slides dry at - 20°C for a long conservation or at room temperature for a short storage. Place the hybridized slides at -20°C and store in dark. The storage period should not exceed 6 months. 68°C 0.3%NP-40/0.4 × In SSc solution,shake for 10 ~ 20 seconds, remove the cover glass and soak for 2 minutes. Place the slide in deionized water at 37 °C and soak it for 1 minute. Dry the slide naturally in the dark and then re dye it. Use the correct filter sets to observe the probe fluorescence. For details, please contact Gene Bio Solution, Technical Service Department. |
