Product Cat. No.: GBS-045
For Research Use Only
1p/19q deletion probe reagent.
10 Tests/box
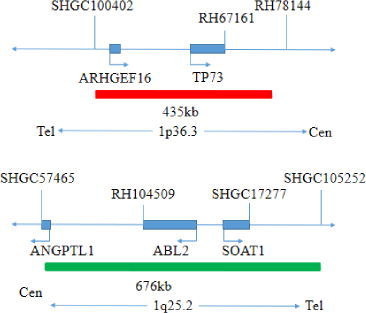
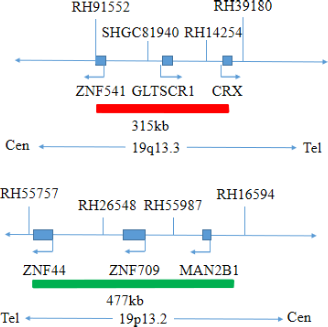
This kit uses orange fluorescent probes 1p36 and 19q13, green fluorescent probes 1q25 and 19p13 to bind 1p/19q probe to the target detection site by in situ hybridization.
The kit consists of 1p36/1q25 dual-color probe and 19q13/19p13 dual-color probe as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| 1p36/1q25 dual color probe | 100μL/Tube | 1 | 1p36 orange probe ; 1q25 green probe |
| 19q13/19p13 dual color probe | 100μL/Tube | 1 | 19q13 orange probe ; 19p13 green probe |
Keep sealed away from light at -20oC±5oC. The product is valid for 12 months. Avoid unnecessary repeated freezing and thawing thatshould not exceed 10 times. After opening, within 24 hours for short-term preservation, keep sealed at 2-8oC in dark. For long-term preservation after opening, keep the lid sealed at -20oC±5oC away from light. The kit should be shipped below 0°C.
Fluorescence microscopy imaging system including fluorescence microscopy and filter sets suitable for DAPI (367/452), Green (495/517), and Orange (547/565).

Negative: 2 Orange-red (2R) ; 2 Green (2G)

Positive: 1 Orange-red (1R) ; 2 Green (2G)---- 1p36/1q25 point-out, 1p36 missing.

Positive: 1 Orange-red (1R) ; 2 Green (2G)------ 19p13/19q13 point-out, 19q13 missing.

At Genebiosolution, our commitment lies in crafting cutting-edge solutions for researchers, clinicians, and diagnostic laboratories seeking excellence in immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). With a focus on quality, reliability, and accuracy, our products empower advancements in molecular diagnostics and research methodologies.