Product Cat. No.: GBS-047
For Research Use Only.
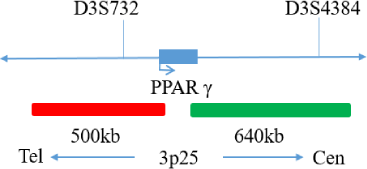
PPARγ(3p25) gene break apart probe reagent.
10 Tests/box.
The reagent carries out in situ hybridization staining on the basis of routine staining to provide doctors with auxiliary information for diagnosis. The test results are only for clinical reference and should not be used as the only basis for clinical diagnosis. Clinicians should comprehensively judge the test results in combination with the patient’s condition, drug indications, treatment response and other laboratory test indicators.
Fluorescence in situ hybridization is a technique for directly observing specific nucleic acids in cells. According to the principle of base complementary pairing, the specific probe is complementary to the target sequence in the cell. Due to the fluorescence of the probe, the gene state of the hybrid probe and the target sequence can be clearly observed under the fluorescence microscope under the appropriate excitation light.
The kit consists of PPARγdual color probe as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| PPARγ dual color probe | 100μL/Tube | 1 | PPARγ Orange probe ; PPARγ Green probe |
Keep sealed away from light at -20°C±5°C. The product is valid for 12 months. Avoid unnecessary repeated freezing and thawing that should not exceed 10 times. After opening, within 24 hours for short-term preservation, keep sealed at 2-8°C in dark. For long-term preservation after opening, keep the lid sealed at -20°C±5°C away from light. The kit is transported below 0°C.
Fluorescence microscopy imaging systems, including fluorescence microscopy and filter sets suitable for DAPI (367/452), Green (495/517), and Orange (547/565).

Negative : 2 fusion

Positive : 1 orange 1 green 1 fusion

At Genebiosolution, our commitment lies in crafting cutting-edge solutions for researchers, clinicians, and diagnostic laboratories seeking excellence in immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). With a focus on quality, reliability, and accuracy, our products empower advancements in molecular diagnostics and research methodologies.