Product Cat. No.: GBS-304
For Research Use Only.
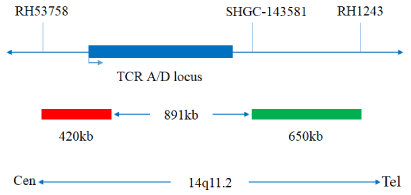
TRA/TRD(14q11) gene break apart probe reagent
10 Tests/box
This kit performs fluorescence in situ hybridization staining based on conventional staining, and provides auxiliary information for diagnosis for physicians. The test results are for clinical reference only and should not be used as the sole basis for clinical diagnosis. Clinicians should make comprehensive judgments on the test results based on factors such as the patient’s condition, drug indications, treatment response and other laboratory test indicators.
Fluorescence in situ hybridization is a technique for directly observing specific nucleic acids in cells. According to the principle of base complementary pairing, the specific probe is complementary to the target sequence in the cell. Due to the fluorescence of the probe,the gene state of the hybrid probe and the target sequence can be clearly observed under the fluorescence microscope under the appropriate excitation light.
The kit consists of TRA/TRD dual-color probes, as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| TRA/TRD dual color probe | 100μL/Tube | 1 | TRA/TRD orange probe ; TRA/TRD green probe |
Keep sealed away from light at -20°C±5°C, and the validity period is 12 months. After the cover is opened, it can be sealed and stored in 2~8℃ away from light within 24 hours. After the cover is opened, it should be sealed and stored in -20±5℃ away from light for a long time. Transport with temperature below 0℃.
Fluorescence microscopy imaging systems, including fluorescence microscopy and filter sets suitable for DAPI (367/452), Green (495/517),and Orange (547/565).
Cell:
1. Take 1-3ml of heparin sodium anticoagulant bone marrow cells.
2. Sample preservation: Fresh bone marrow specimen without fixation (preserved at 2-8°C for no more than 24 hours). After fixation, the cell suspension can be preserved at -20±5°C for no more than 12 months; the prepared cell slide can be preserved at -20±5°C for no more than 1 month. When the storage temperature of the sample is too high or too low, the cell suspension is volatilized excessively or polluted,the sample cannot be used for detection.
Tissue:
1. Applicable specimen types: Paraffin-embedded specimens from surgical excision or biopsy.
2. The tissue should be fixed with 4% neutral formaldehyde solution within 1 hour after isolation. After tissue fixation, it is routinely dehydrated and embedded in paraffin.
1. Related reagents
The following reagents are required for the experiment but not provided in this kit
2. Sample processing before hybridization
Cells sample:
1. Sample collection: Take 3mL of anticoagulated bone marrow cell samples.
2. Cell harvesting: Place 3 mL of anticoagulated peripheral blood in a 15 mL centrifuge tube, centrifuge at 500g for 5 min, carefully discard the supernatant, and resuspend about 500μL of the residue.
3. Cell washing: Add 5 mL of 1×PBS buffer, mix and resuspend the cell pellet, centrifuge at 500g for 5 min, carefully discard the supernatant,and resuspend the cells with about 500μL of the residue; repeat 1 time.
4. Cells hypotonicty: Add 10mL of hypotonic solution pre-warmed to 37°C and place in an water bath at 37°C for 15-20min.
5. Cells pre-fixation: Pre-fix the cells by adding 1mL (10% by volume) of fixative solution to the cell suspension after the completion of hypotonic osmosis. Gently pipette, mix and centrifuge for 5 min at 500g, discard the supernatant, and resuspend about 500μL of the residue.
6. Cell fixation: Slowly add 10mL of fixative solution to the cell suspension at room temperature for 10 min, centrifuge at 500g for 5 min, and resuspend the cells with about 500μL of the residue; repeat once (the cells may be fixed several times until the cells pellet is washed and cleaned).
7. Cell suspension preparation: Pipet the supernatant and add the appropriate amount of fixative solution to prepare the appropriate cell suspension concentration.
8. Slides preparation: Pipet 3-5μl of cell suspension drop onto the slides,put at 56°C for 30min.
9. Pretreatment: At room temperature, rinse the glass slides twice with 2xSSC (pH 7.0) solution for 5min each time.
10. Dehydration: Place the glass slides in 70% ethanol, 85% ethanol and 100% ethanol and dry for 2 minutes.
Tissue sample:
Baking: Slides heating at 80°C for 30min or 65°C for 2h or overnight.
Dewaxing: According to the customer laboratory protocol (Commonly with Xylene for 15min).
Hydration: Take out the slides and put them respectively into 100%, 85% and 70% EtOH at room temperature for 3 minutes each.
Take out the slides, and immerse them in deionized water for 3 minutes. Remove the excess of water on the slides by air-drying.
Permeation: Immerse the slides in deionized water at 100°C and boil continuously for 20-40 minutes (Conventional 20min). Remove the excess of water on the slides by air-drying.
Digestion: Protease enzymic digestion at 37°C for 10-40 minutes. Mix the protease work buffer (50mmol HCl) and the 10x protease solution (Pepsin concentration 5%) in a proportion of 9:1 to prepare the enzymatic digestion solution.
Washing: Wash with 2xSSC at room temperature for 5 minutes.
Dehydration: Take out the slides and dehydrate in 70%, 85%, and 100% gradient ethanol at room temperature for 2 minutes each time.
Remove the excess of EtOH solution on the slides by air-drying.
3. Denaturation and Hybridization
The following operations need to be carried out in the darkroom.
Cell sample:
Tissue samples:
4. Washing
The following operations need to be carried out in the darkroom.
5. Counterstaining
The following operations should be performed in a darkroom
10μL DAPI compound dye is dropped in the hybridization area of the glass slide and immediately covered. The suitable filter is selected for glass slide observation under the fluorescence microscope.
6. FISH results observation
Place the stained sections under a fluorescence microscope and the cells area is first confirmed under a low magnification objective (10x); under magnification objective (40x) a uniform cells distribution is observed; then the nucleus size uniformity, nuclear boundary integrity,DAPI staining uniformity, no nuclei overlapping, cells clear signal are observed in the high magnification objective (100x).

Negative: 2 fusion

Positive: 1 orange 1 green 1 fusion
1. Please read this manual carefully before testing. The testing personnel shall receive professional technical training, and the signal counting personnel must be able to observe and distinguish orange and green signals.
2. When testing clinical samples, when the hybridization signal counting is difficult and the sample is not enough to repeat the retest, or the cell volume is not enough for analysis, the test will not provide the test results.
3. DAPI counterstaining agent used in this experiment has potential toxicity or carcinogenicity, so it is necessary to operate in the fume hood,wear masks and gloves to avoid direct contact.
4. All chemicals are potentially dangerous. Avoid direct contact. Used kits are waste and should be properly disposed off.

At Genebiosolution, our commitment lies in crafting cutting-edge solutions for researchers, clinicians, and diagnostic laboratories seeking excellence in immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). With a focus on quality, reliability, and accuracy, our products empower advancements in molecular diagnostics and research methodologies.