FAQ'S
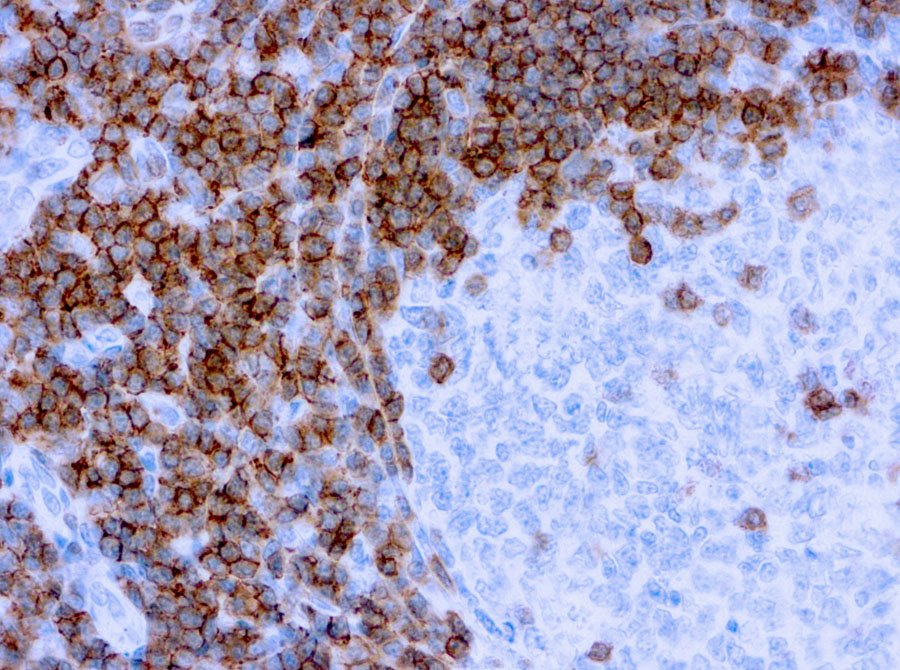
Primary antibodies are used in Immunohistochemistry (IHC) to detect specific proteins within tissue samples. They bind directly to the target protein, allowing researchers to visualize where and how much of the protein is present. This helps in studying tissue structure, understanding disease mechanisms, and identifying abnormal cells. IHC is often used in both research and clinical diagnostics. These antibodies enable detailed mapping of protein expression and cellular localization, which is critical in detecting disease biomarkers. Their use is essential in assessing disease progression, especially in cancer and autoimmune conditions. Primary antibodies are often paired with secondary antibodies for signal amplification and clearer visualization.
Researchers select primary antibodies based on the target protein, species compatibility, application type (like IHC or Western blot), and antibody specificity. The choice also depends on whether the antibody is monoclonal or polyclonal. Careful validation is essential to ensure the antibody delivers reliable and reproducible results in the study. Researchers also review scientific literature and data sheets to ensure the antibody has been successfully used in similar applications. Factors such as host species, clonality, and epitope recognition are carefully reviewed. Validated antibodies contribute to consistent experimental outcomes and help reduce variability in research.
Yes, primary antibodies play a crucial role in cancer diagnostics. They help identify specific markers or proteins that are overexpressed or mutated in cancer cells. This allows doctors to classify tumor types, determine prognosis, and guide treatment decisions. Primary antibodies are widely used in pathology labs during biopsy analysis and cancer screening.
Their use enhances precision in identifying tumor subtypes, such as hormone receptor status in breast cancer. IHC panels using multiple antibodies offer deeper insight into tumor behavior. Moreover, they aid in monitoring treatment response and detecting recurrence in follow-up biopsies.
Primary antibody performance can be influenced by antibody quality, concentration, incubation time, and sample preparation. Other factors like temperature, buffer composition, and the presence of background staining also play a role. Proper optimization and validation are crucial for achieving accurate and consistent results. Storage conditions and shelf-life can impact stability and efficacy. Variations in tissue fixation methods may alter epitope accessibility, affecting antibody binding. It’s also important to use appropriate positive and negative controls to evaluate performance and troubleshoot inconsistent results.
Both monoclonal and polyclonal antibodies have advantages in IHC, depending on the application. Monoclonal antibodies offer high specificity to a single epitope, making them ideal for consistent results. Polyclonal antibodies, on the other hand, can detect multiple epitopes, offering stronger signals. The choice depends on the target and the research needs. Monoclonal antibodies are preferred when high reproducibility is required across batches. Polyclonal antibodies may be more effective for detecting low-abundance targets or conformational variants. Researchers often test both types during assay development to determine which yields the best signal-to-noise ratio.
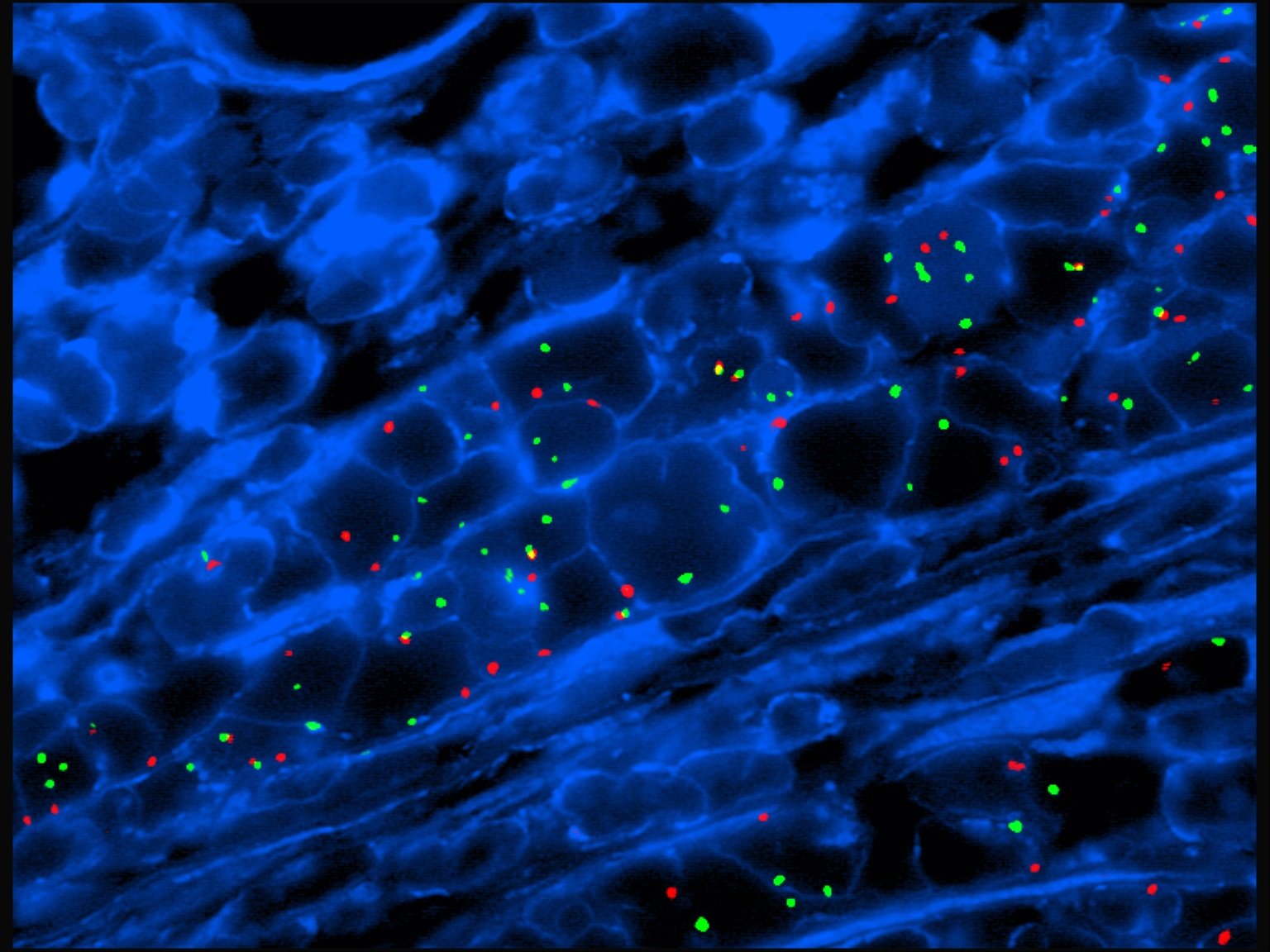
FISH (Fluorescence In Situ Hybridization) probes are used to detect and locate specific DNA or RNA sequences in cells. They help identify genetic abnormalities such as deletions, duplications, or translocations. FISH is especially valuable in diagnosing genetic disorders, cancers, and other chromosomal changes. It provides precise and reliable results in clinical diagnostics. These probes glow under a microscope, making it easy to see the genetic changes. Doctors can use the results to guide treatment plans or confirm a diagnosis. FISH can be done quickly and is often used when time matters.
FISH probes can help diagnose a variety of conditions, including genetic disorders like Down syndrome, cancer types such as breast or leukemia, and chromosomal abnormalities like deletions or duplications. They are also used in prenatal testing and to detect gene rearrangements in tumor samples. FISH is a valuable tool for early and accurate diagnosis. It is commonly used when other tests are unclear or need confirmation. The clear, colorful results make it easier to spot changes. Because it works at the DNA level, FISH gives deep insights into a person’s genetic health.
FISH bio probes give very accurate results when carefully made and correctly used. These probes are great at finding changes in genes, such as extra copies, missing parts, or swapped sections. The results are seen under a microscope, making it easy to spot these changes in chromosomes. Accuracy also depends on how good the sample is and how skilled the lab team is. Proper storage of the probes and samples ensures clear signals. Using high-quality reagents and controls helps avoid false results. Skilled technicians are trained to spot even small changes. That’s why FISH is trusted in labs worldwide.
Yes, FISH probes can be customized to target specific genes or chromosomal regions of interest. Custom probes are especially useful in research or rare disease diagnostics where standard probes are not available. Labs can design these probes to match the exact sequence needed, ensuring precise hybridization and accurate results in gene analysis. Customization is helpful when studying new or less common genetic traits. Scientists can focus on a specific area of interest that standard tests might miss. This allows for more flexibility and innovation in diagnostics. Custom FISH probes help solve complex genetic puzzles.
FISH testing can be performed on a variety of sample types including tissue sections, blood samples, bone marrow, amniotic fluid, and cultured cells. The choice depends on the condition being tested. For cancer diagnosis, biopsy tissue is often used, while for genetic testing, blood or prenatal fluid samples are common. Proper sample preparation is key for accurate results. Each sample type may require a different process before testing. For example, cells need to be spread and fixed correctly on slides. Fresh samples give clearer results than degraded ones. Following the right steps helps the FISH probes bind correctly.